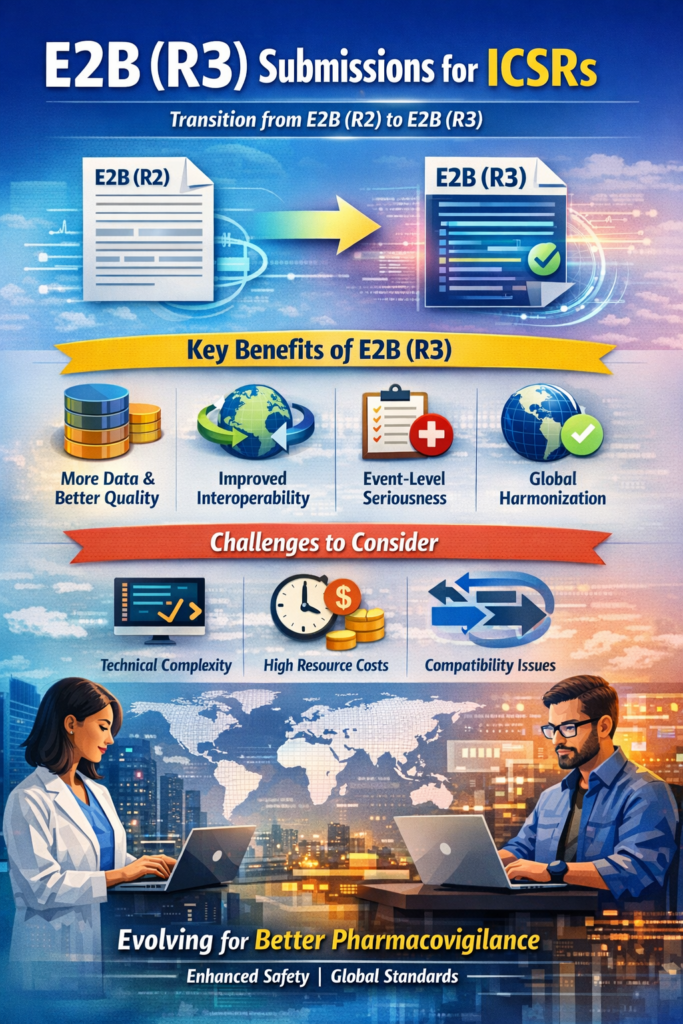
Learn about E2B (R3) submissions for ICSRs.
This summary outlines the key points regarding the transition from the E2B(R2) to the E2B(R3) standard for Individual Case Safety Reports (ICSR) submissions. Overview of ICSR E2B R2 vs. R3 The E2B guideline, established by the International Council for Harmonization (ICH), provides the data elements and message specifications for the electronic transmission of ICSRs. While […]
Most Common Mistakes by Newbies entering Pharmaceuticals
All of the above can be avoided and improved upon using these five strategies. To learn more from related topics, please visit our website or newsletter at https://medipharmsolutions.com/newsletter/
The Future of Drug Safety Is Hybrid: Human Expertise Powered by AI
The integration of AI into pharmacovigilance is more than a technological trend — it’s a fundamental shift that impacts workflows, capabilities, and most importantly, careers. Whether you are an early-career professional, an experienced PV specialist, or a leader shaping organizational strategy, understanding this intersection is essential for future success. Why AI Matters in Pharmacovigilance Pharmacovigilance […]
The Harsh Truth About Building a Career in the Pharmaceutical Industry
In the pharmaceutical industry, few careers fail because of a lack of intelligence, education, or ambition. More often, careers stall because people wait too long on the sidelines—waiting for clarity, confidence, or the perfect opportunity. It means engaging actively with your career, taking ownership of opportunities, and learning through real-world experience. The difference between professionals […]
Cracking the Pharmacovigilance Interview: What Hiring Managers Really Look For
Preparing for an interview in the field of Drug Safety and Pharmacovigilance requires far more than memorizing answers or reviewing common interview questions. This is a highly regulated, patient-centric discipline where employers are not only assessing your technical knowledge but also your mindset, ethical grounding, attention to detail, and ability to function within complex global […]
A Day in the Life of Drug Safety Associate
Catching a glimpse at a regular 9 to 5 working day of a Drug Safety Associate. CASE PROCESSING A Drug Safety Associate is required right from triage, book-in to case submissions – all the parts of case processing. The role is often more streamlined depending on the magnanimity of the Pharmacovigilance team in an organization […]