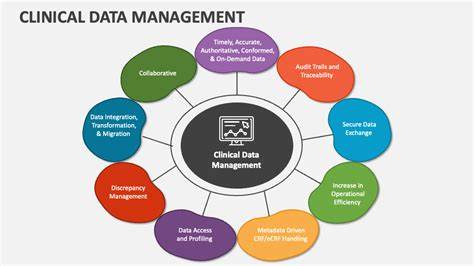
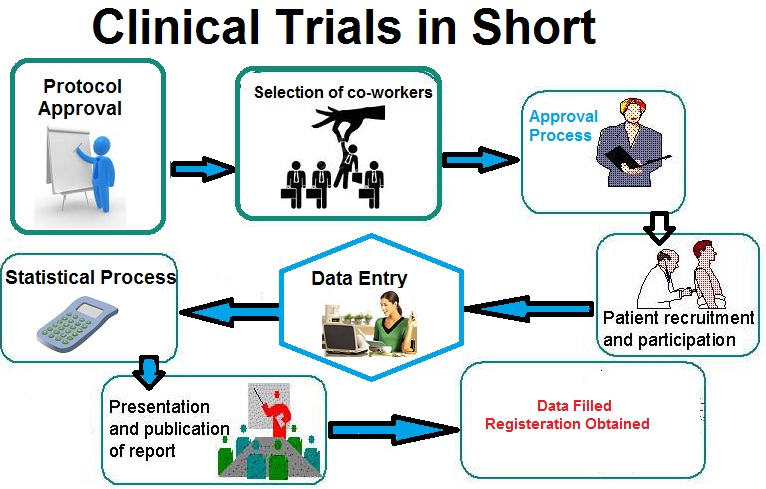
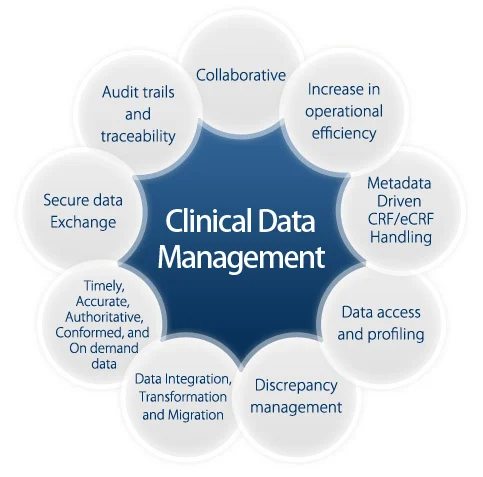
Handling regulatory inspections in pharmacovigilance can be challenging, but organizations can gain valuable insights to improve their processes. Here are key lessons learned from successful inspection experiences: 1. Preparation...
Handling regulatory inspections in pharmacovigilance can be challenging, but organizations can gain valuable insights to improve their processes. Here are key lessons learned from successful inspection experiences: 1. Preparation...